Water in the air is a funny thing. Freeze it the right way, and it comes down in flakes so fluffy that snowboarding feels like flying. Freeze it the wrong way, and it shuts down London Heathrow airport for days when you are trying to get back to New York for Christmas.
With so many thousands of us stranded here in Europe, it’s easy to focus on the water in the air you see. But more intriguing still is the water in the air you don’t see — which once understood, helps explain everything from why we get sick in the winter to why global warming is dangerous.
I stumbled upon this little known fact last winter, watching my cheap Chinese humidifier spray a pathetic strand of fog into the dry Beijing air. Was this thing actually accomplishing anything?, I pondered. I mean, seriously, was that litre of water really going to make a difference? How much water is there in my room anyway — a mililiter? a hundred liters? I had not the foggiest idea.
Before I tell you the answer, take a guess. We don’t usually know this number because humidity gets quoted in relative terms (e.g. 70%), not absolute ones (i.e. grams per cubic meter). Which makes sense: how dry the air feels reflects how readily water evaporates off our skin (and from our sore winter throats). That in turn depends on how saturated the air is, not how much absolute water is in it.
Ok, here’s the answer: air at room temperature is saturated when it holds about 20 grams of water per cubic meter (20 teaspoons if you don’t do grams). So my 4m x 4m x 3m meter room could hold about 1,000 grams or 1 litre of water at saturation, so hmm… yep, pumping a quarter litre of fog into the air would raise relative humidity from a dry 25% to a comfortable 50%. Humidifiers work!
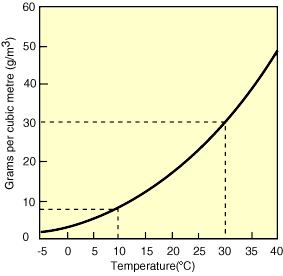
But promoting humidifiers wasn’t what inspired this Aabservation. (Though I am excited to now have an Amazon referrals sidebar on my blog, which you are welcome to play with…) What I also stumbled upon that dry Beijing morning was that 20 grams is only the quantity of water that saturates air at room temperature. Drop the temperature to 10 C (50 F), and air can only hold 9 grams of water — half as much as at 20 C.
Which is why winter air indoors is so dry. Think about it: even if snowing outside (100% humidity), at 0 C air contains less than 4 grams of water per cubic meter. Bring that same dry air inside, heat it up to room temperature (which you’ll remember can hold 20 grams of water), and 4 grams becomes just 1/5 of the total amount of water the air can hold — or an uncomfortable 20% humidity.
No wonder we get sick and our throats feel dry in the winter. Indeed, low absolute humidity is a good predictor of flu transmission, according to a recent study.*
Now think more macro, and you’ll understand how even a few degrees increase in global climate can have a powerful impact on storm intensity. Air at 30C (86F) can hold a whopping 30 grams of water, 32% more water than air just 5C (9F) cooler (see table). That’s 32% more actual water that can then be absorbed into storm clouds and dumped on your beach!
Of course, I am clearly not a meteorologist and this Aabservation doesn’t take into account factors like atmospheric pressure and cloud formation patterns. But hey — this water in air thing is a kind of intriguing, no?
To close, here’s a picture from the Australian government that explains the relationship between humidity and temperature beautifully:
That’s enough about water in the air for one night. Now back to contemplating water in the airport — you know, the stuff that froze Heathrow to a standstill and has pushed my December 21st flight to New York back to December 25th.
On the bright side, maybe I’ll bump into Santa in the air that day. Maybe he’s even bought me a humidifier.
Merry Christmas from London,
Liz
